IJN Research Management System (iRMS) for Institut Jantung Negara
Digitizing healthcare research workflows for one of Southeast Asia’s premier heart institutions.
Empowering Medical Research with Scalable Digital Infrastructure
Client: Institut Jantung Negara (National Heart Institute), Kuala Lumpur, Malaysia
Sector: Healthcare, Medical Research
Scope: Custom Health Solution for Research Management
Project Name: iRMS – IJN Research Management System
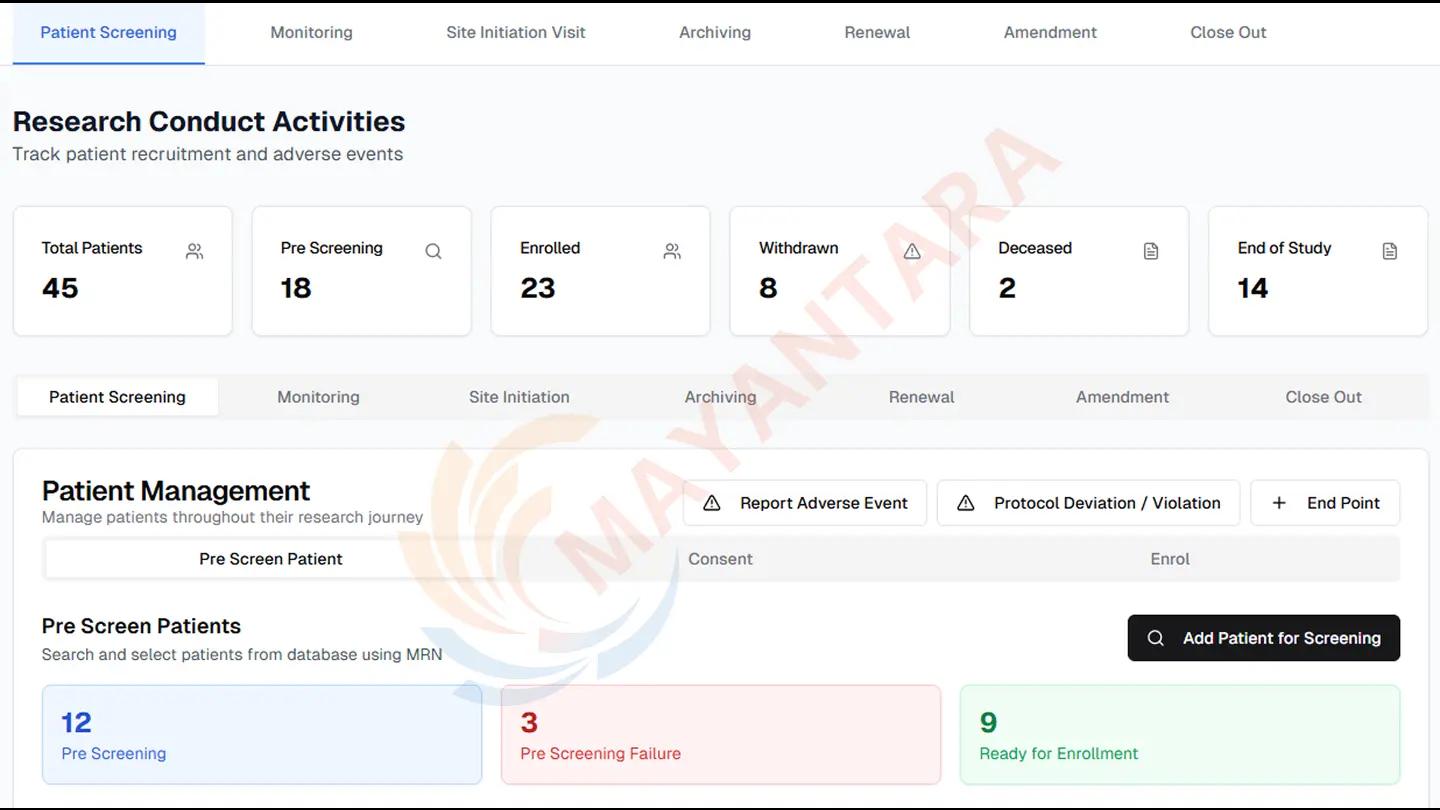
🔍 The Challenge
As a leading cardiac institution, Institut Jantung Negara (IJN) manages a significant volume of clinical and academic research. Their existing research workflows were largely manual, fragmented, and lacked real-time coordination between investigators, sponsors, ethics reviewers, and research committees.
🚀 The Digital Health Solution by Mayantara
Mayantara designed and implemented a robust and modular digital research health solution known as iRMS (IJN Research Management System). This tailored platform centralizes the research lifecycle — from proposal to publication — ensuring visibility, accountability, and ethical compliance.
Core Features Implemented:
Centralized research project registration and approval
End-to-end research data and document tracking
Ethics review workflows and risk assessments
Sponsor and grant application management
Integration with IJN’s internal regulatory processes
🧠 Compact List of Modules
iRMS is built as a modular platform. The primary components include:
Core Operations: Dashboard, Workflow, Meetings, Human Subject
Project Lifecycle: Registration, Conduct, Output, Publications
Governance: Risk Assessment, Ethics Review, Agreements
Funding & Support: Research Grant, Sponsors, Support Services
These modules are aligned with international standards and support digital governance in research operations.
📊 The Outcome
The iRMS platform has enhanced visibility into research activities at IJN, reduced processing times, and fostered a data-driven research culture. With full audit trails, status monitoring, and approval routing, the platform represents a milestone in digital transformation for clinical research.
Their Experience Our Pride
These voices reflect our commitment to solving real problems with purposeful technology that works in the field, not just on paper.
Let’s start something impactful.